Link to our unpublished work on bioRxiv
Link to all publications
Selected papers
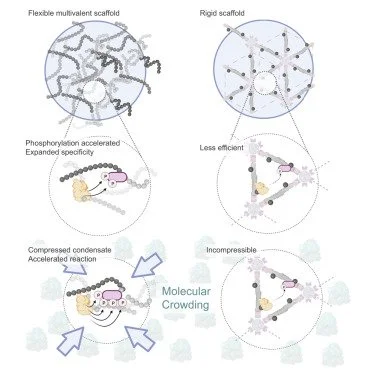
Condensed-phase signaling can expand kinase specificity and respond to macromolecular crowding Sang, D., Shu, T., Pantoja, C. F., de Opakua, A. I., Zweckstetter, M., & Holt, L. J. (2022). Molecular Cell, 82(19), 3693-3711.
Understanding signaling dynamics within endogenous mesoscale biomolecular condensates is difficult due to intricate connections between structures and functions. Here, we adopted a synthetic biology approach to study this relation by constructing dynamic kinases phosphoregulations within synthetic condensates in S. cerevisiae yeast cells, where we found increased phosphorylation activity and broadened kinase specificity. This is not only due to high client concentrations within the condensates but also the accessibility of excess client-binding sites with a flexible scaffold. Phosphorylation within condensate also responds to macromolecular crowding in cells, thus allowing it to act as a sensor for detecting changes in cytoplasm biophysical properties. Finally, we demonstrated that phase separation can also accelerate Alzheimer’s-associated phosphorylation.
SWI/SNF senses carbon starvation with a pH-sensitive low-complexity sequence Gutierrez, J. I., Brittingham, G. P., Karadeniz, Y., Tran, K. D., Dutta, A., Holehouse, A. S., ... & Holt, L. J. (2022). SWI/SNF senses carbon starvation with a pH-sensitive low-complexity sequence. Elife, 11, e70344.
This paper elucidates the molecular mechanisms of pH sensing. In Saccharomyces cerevisiae, nucleocytoplasmic pH oscillation is required for the transcriptional response to carbon starvation and SWI/SNF chromatin remodeling complex is a key mediator of this process. SNF5 QLC acts as a pH sensor and pH changes are a second messenger for transcriptional reprogramming in response to carbon starvation.
mTORC1 Controls Phase Separation and the Biophysical Properties of the Cytoplasm by Tuning Crowding Delarue M., Brittingham G.P., Pfeffer S., Surovtsev I.V., Pinglay S., Kennedy K.J., Schaffer M., Gutierrez J.I., Sang D., Poterewicz G., Chung J.K., Plitzko J.M., Groves J.T., Jacobs-Wagner C., Engel B.D., Holt L.J. Cell. 2018 Jul 12
This paper shows that the central growth regulator mTORC1 determines the physical properties of the cytoplasm! By modulating the concentration of ribosomes through production and autophagy, the rates of diffusion of everything in the cell that is > 20 nm diameter can be varied 2-fold in yeast and 20% in humans. Therefore, all of the second-order rate constants for any reasonably-sized complex in the cell is not constant. Also, phase separation is substantially regulated by this crowding regulation.
SCWISh network is essential for survival under mechanical pressure Delarue M., Poterewicz G., Hoxha O., Choi J., Yoo W., Kayser J., Holt L.J.*, Hallatschek O.* PNAS 2017 Dec 19
We know quite a bit about how cells sense tensile stresses, but almost nothing about how cells detect and respond to mechanical compressive stress (pressure). All of the cells in our body are under pressure. We should probably figure this out. This paper describes the SCWISh pathway, required for cells to survive when growing under pressure! At the top of the pathway is a mucin. Mucins are frequently misregulated in cancer. We think these sensors will play an important role in the adaptation of cancer to new mechanical conditions.
Ancestral resurrection reveals evolutionary mechanisms of kinase plasticity Howard, C., Hanson-Smith, V., Kennedy, K.J., Miller, C.J., Lou, H.J., Johnson, A.D., Turk, B., and Holt, L.J. Elife 2014 3.
Amazingly, we can reconstruct and resurrect kinases from >2 billion years ago and determine their specificity! The deep ancestors of Cdk1, MAP kinases and Ime2 reveal that the gatekeeper residue helps determine specificity. This paper also suggests that kinase networks can tolerate new information, thus providing a pathway for dramatic rewiring of the cell's circuitry.
Global Analysis of Cdk1 Substrate Phosphorylation Sites Provides Insights into Evolution. Holt, L.J., Tuch, B., Villen, J., Johnson, A., Gygi, S., and Morgan, D. Science 2009
We combined the "Shokat" analog-sensitive kinase approach with SILAC quantitative mass spectrometry (with the Gygi lab) to discover >300 Cdk1 substrates in S. cerevisiae. Evolutionary analysis revealed that these sites, while often conserved for >1 billion years (bottom), are very rarely conserved in terms of their precise position (top). This work suggests that phosphorylation networks are quite evolvable. Biological information systems can change readily to adapt to new challenges.
Positive feedback sharpens the anaphase switch. Holt, L.J., Krutchinsky, A.N., and Morgan, D.O. Nature 2008
Selected bioRxiv preprints
1. Increased mesoscale diffusivity in response to acute glucose starvation Xie, Y., Gresham, D., & Holt, L. J. bioRxiv (2023) doi.org/10.1101/2023.01.10.523352
3. nucGEMs probe the biophysical properties of the nucleoplasm Shu, T., Szórádi, T., Kidiyoor, G. R., Xie, Y., ... & Holt, L. J. bioRxiv (2021) doi.org/10.1101/2021.11.18.469159
Positive feedback can generate switch-like behaviors where systems abruptly and irreversibly transition from one state to another (bistability). We found a positive feedback look involving Cdk1, Cdc14, Securin, Separase and the Anaphase Promoting Complex (some of the tastiest spices in the soup of regulators that is the cell cycle). This feedback loop ensures synchronous chromosome segregation at anaphase onset and improves the fidelity of cell division.
2. Condensation of LINE-1 is required for retrotransposition Sil, S., Boeke, J.D., Holt, L.J. bioRxiv (2022) doi.org/10.1101/2022.04.11.487880